Coupled Mathematical Model Examines Host-reservoir Transmission of Ebola
Ebola virus disease (EVD) is an infectious and often deadly viral hemorrhagic fever that affects humans and primates. It is caused by an orthoebolavirus (see Figure 1) and primarily found in communities in sub-Saharan Africa [1]. Early symptoms include fever, sore throat, and muscle pain, followed by vomiting, diarrhea, rash, and internal and/or external bleeding. EVD is often fatal, with an average mortality rate of 50 percent [2].
The first recorded cases of EVD occurred in 1976 during two simultaneous outbreaks in Sudan and the Democratic Republic of the Congo [2]. In 2014 and 2015, a significant and complex outbreak—the deadliest on record—plagued Guinea, Liberia, and Sierra Leone; it spread to seven additional countries, resulted in more than 11,000 deaths, and left a devastating economic toll in its wake [2].
Fruit bats of the Pteropodidae family are the probable natural reservoir species for EVD, as they seem to carry the virus without presenting symptoms. Transmission to humans can occur in several ways, such as direct contact with blood secretions, organs, or other bodily fluids of infected animals (bats, chimpanzees, gorillas, etc.). Other sources of transmission include indirect environmental contamination and bat-to-human spillover events.
While existing mathematical models of EVD focus primarily on human-to-human transmission, a truly realistic model should combine all host sources. During a contributed presentation at the Third Joint SIAM/CAIMS Annual Meetings, which are currently taking place in Montréal, Québec, Canada, Herve Michel Djouosseu Tenkam of North-West University in South Africa presented a novel two-host mathematical model of EVD to assess the reservoir bat species’ impact on disease dynamics and spread. His unique model accounts for both bats and humans, incorporates EVD spillover potential by switching the zoonotic pathogen from the bat to the human population, and utilizes advanced analytical techniques for stability analysis. “The goal is to assess the reservoir impact on the transmission dynamics of EVD,” Djouosseu Tenkam said.
Djouosseu Tenkam’s methodology involves three distinct steps: (i) Model the transmission dynamics in the bat population, (ii) model the transmission dynamics in the human population, and (iii) combine both models to assess the true complex dynamics of transmission. He began with the bat submodel and explained his hypothesis for this component. “Bats catch the infection by direct contact with an infected bat at rate \(\beta_4\), or by indirect contact with the viruses that are shed in the environment when they eat contaminated fruits or vegetables,” Djouosseu Tenkam said. He also assumes that infected bats do not die from EVD; instead, they carry the virus throughout their lives and shed it into the environment at a rate \(\lambda_b\). And since there is no intrinsic growth for free-living EVD viruses in the environment—or for all free-living viruses in general—Djouosseu Tenkam postulates that such viruses either deplete naturally or through environmental decontamination techniques at a constant rate \(\eta\). Finally, he considers bilinear incidence rates for both direct and indirect transmissions.
Given these hypotheses, Djouosseu Tenkam presented his simple three-compartment bat submodel with compartments for susceptible bats \((S_b)\); infected bats \((I_b)\), which have no option for recovery and instead maintain lifelong infections; and environmental viruses \((P)\), which occur when infected bats deposit the virus into their environments in an amount then decays overtime. Transmission routes include direct bat-to-bat contact \((\beta_4)\) and indirect contact from environment to bat (\(\lambda_b)\).
Next, Djouosseu Tenkam introduced the human epidemic submodel, an epidemic model with five compartments: susceptible \((S)\), infected \((I)\), recovered \((R)\), deceased \((D)\), and environmental viruses \((P)\). Here, transmission routes include human-to-human transmission \((\beta_1, \beta_2)\) and indirect environmental contact \((\lambda)\). Even deceased individuals can deposit an indirect infection in the environment or cause direct contamination after death.
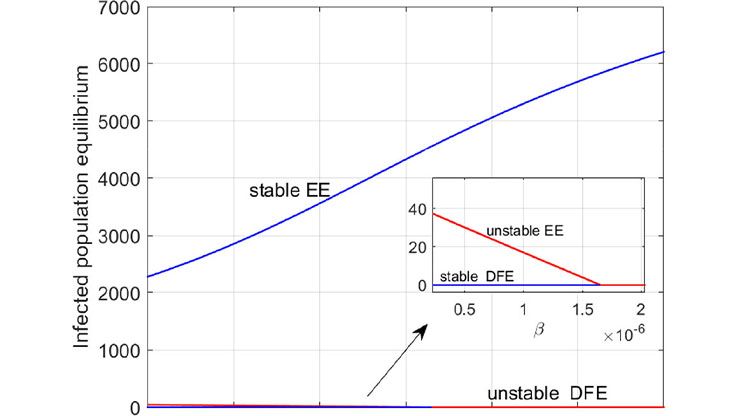
Djouosseu Tenkam combined these two separate models into a larger, coupled bat-human spillover model with a variety of different transmission routes (see Figure 2). He found the basic reproduction number \((R_0)\) for the bat submodel, human submodel, and coupled model, then presented stability results for the coupled model. For instance, he found that a disease-free equilibrium with global asymptotic stability exists when \(R_0 \le 1\); the equilibrium is unstable when \(R_0 >1\). An endemic equilibrium exists uniquely with global asymptotic stability when \(R_0 > 1\). In the absence of a spillover event, the corresponding \(R_0\) of the coupled model implies that the spillover phenomenon accelerates the spread of EVD.
At present, there is no real “cure” for EVD beyond general treatment and supportive care. Djouosseu Tenkam thus concluded his presentation with a discussion of control strategies (e.g., vaccination) that could target susceptible individuals and prevent an outbreak. Computation of the target reproduction number—a function of \(R_0\)—offers a better understanding of the requirements for disease eradication given targeted vaccine treatment for susceptible individuals. Numerical results via MATLAB simulation affirm that a high spillover rate leads to an endemic state, while a low spillover could result in disease extinction. “This proves that spillover events can contribute to speedups in disease outbreak,” he said.
In the future, Djouosseu Tenkam hopes to expand upon this work by exploring multi-patch models, experimenting with additional control strategies like quarantine, and including other animal reservoirs.
References
[1] U.S. Centers for Disease Control and Prevention. (2024, April 23). Ebola disease basics. Retrieved from https://www.cdc.gov/ebola/about/index.html.
[2] World Health Organization. (2025). Ebola virus disease. Retrieved from https://www.who.int/health-topics/ebola.
About the Author
Lina Sorg
Managing editor, SIAM News
Lina Sorg is the managing editor of SIAM News.

Stay Up-to-Date with Email Alerts
Sign up for our monthly newsletter and emails about other topics of your choosing.