Heat Exchange and Some Frivolous Aspects of e
Is it possible to heat a glass of \(0 ^\circ \textrm{C}\) milk to \(>50 ^\circ \textrm{C}\) using only the heat from an identical glass of \(100 ^\circ \textrm{C}\) water, thus cooling the water to \(< 50 ^\circ \textrm{C}\)? No heat is exchanged with the outside world, extra containers are available, and heat capacities per unit volume of the water and milk are assumed to be the same.
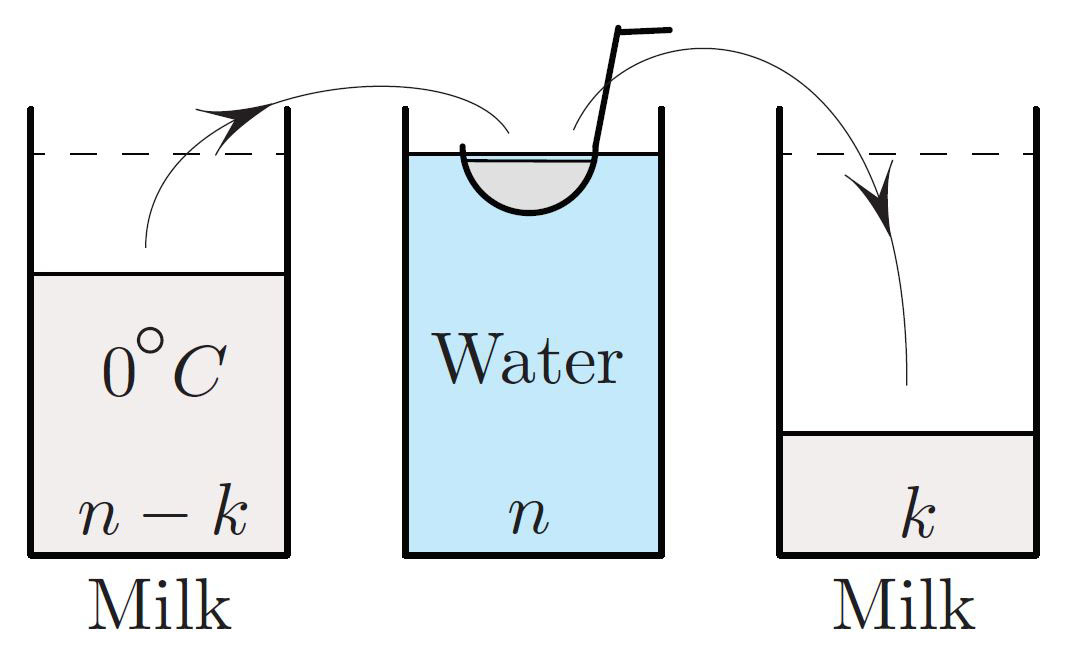
Despite the fact that heat flows “downhill” temperature-wise (the second law of thermodynamics), one can indeed reverse the order of the two liquids’ temperatures, as illustrated in Figure 1. We scoop \(1/n\)th of the milk into a ladle, dip the ladle in the hot water until the temperatures equalize, and dump the warmed milk into the glass on the right. After \(n\) repetitions, all of the milk ends up in the last glass. Dipping the \(0 ^\circ \textrm{C}\) milk ladle in the warm water reduces the water temperature by the same factor on each step:
\[T_{k+1}= \frac{n}{n+1} T_k, \ \ T_0 = 100 ^\circ \textrm{C}, \tag1 \]
since the heat of \(n\) units of water spreads equally among the \(n+1\) units of liquid.
After \(n\) steps,1 with all of the milk in the third glass, the water therefore cools to
\[\frac{100}{\bigl(1+ \frac{1}{n} \bigl) ^n } \approx\frac{100}{e} \approx 36.8 ^\circ \textrm{C};\]
coincidentally, this is the human body temperature. The milk’s temperature is thus \(\approx 63 ^\circ \textrm{C} \), considerably above \(50 ^\circ \textrm{C}\). This is actually the perfect temperature for cooked salmon. To summarize,
\[T_{\rm body}+T_{\rm salmon}\approx 100 ^\circ \textrm{C}\]
and
\[e\approx \frac{100 ^\circ \textrm{C}}{T_{\rm body}}.\]
Even more surprising than the reversal of the order of temperatures is the fact that a near-perfect temperature swap is possible in principle. Biological evolution “invented” the mechanism of such a swap, which may be described in another article.
1 We assume a small ladle, hence a large \(n\).
References
[1] Levi, M. (2012). Why Cats Land on Their Feet: And 76 other Physical Paradoxes and Puzzles. Princeton, NJ: Princeton University Press.
About the Author
Mark Levi
Professor, Pennsylvania State University
Mark Levi (levi@math.psu.edu) is a professor of mathematics at the Pennsylvania State University.
Stay Up-to-Date with Email Alerts
Sign up for our monthly newsletter and emails about other topics of your choosing.



