Innovative Microfluidic Device Design Detects Circulating Tumor Cells
Circulating tumor cells (CTCs) play a critical role in cancer progression because they transform into metastasis agents, which spread from the primary tumor to distant organs via the bloodstream. The subsequent formation of secondary tumors in vital organs significantly impacts patient health and complicates treatment. Given CTCs’ pivotal involvement in cancer metastases, the early detection and precise identification of these elusive cells are imperative objectives in the field of oncology. In addition to revealing disease stage and severity, early CTC detection empowers healthcare professionals to create informed treatment plans with the power to revolutionize cancer care with precise, timely interventions that are tailored to patients’ needs.
Research laboratories employ a variety of techniques that isolate CTCs from blood samples and reflect their significance in the context of cancer [6, 8-10]. These methods have been instrumental in advancing our understanding of the role of CTCs in disease progression. However, the cells’ inherent heterogeneity complicates matters. Current detection methods often isolate all CTCs in a blood sample without distinguishing between distinct subpopulations [3]. Studies have shown that CTC characteristics—including surface markers, genetic mutations, and molecular profiles—exhibit considerable diversity [1, 2, 5, 7]. In fact, specific CTC subpopulations exhibit unique traits and genetic signatures that make them particularly influential in cancer metastasis [4]. Recognizing and isolating these subpopulations will offer deeper insights into the metastatic process and facilitate the development of targeted therapies that are tailored to the cells’ individual characteristics. As such, researchers have proposed innovative detection methods that are rooted in genomic and transcriptomic analysis. Immunofluorescence characterization is particularly invaluable because it both confirms the presence of CTCs and differentiates them from nonspecifically adhered blood cells. However, this procedure is limited by the relatively restricted pool of available biomarkers for labeling and targeting CTCs.
Microfluidic devices have emerged in response to these challenges and are revolutionizing CTC detection. These devices enhance the contact between CTCs and antibodies within three-dimensional channels, thus optimizing detection and isolation through proximity-based magnetic or electric fields. Their construction from transparent materials enables the precise visualization and characterization of small objects—including labeled living cells—and their compact design promotes cost effectiveness and expedites CTC analysis. The field of microfluidics has inspired groundbreaking innovations that overcome challenges in cell separation and CTC identification, further propelling our understanding of the role of these elusive yet pivotal cells in cancer progression.
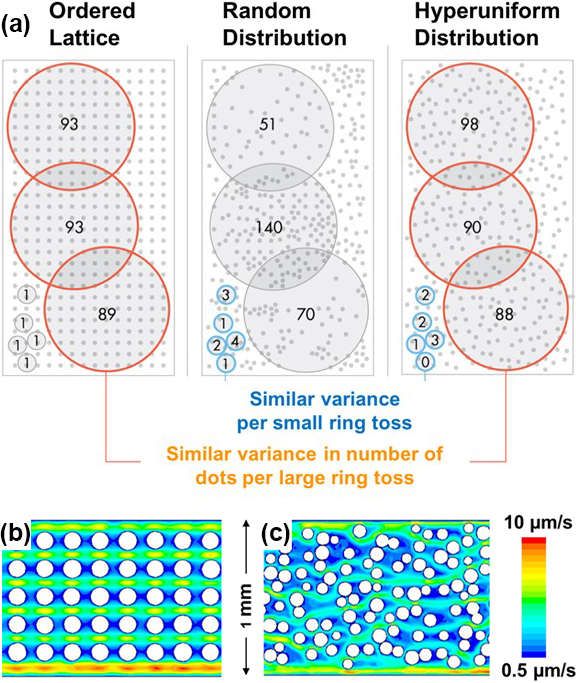
Since conventional microfluidic approaches may harm delicate CTCs through cell permeabilization and fixation, practitioners must advance label-free techniques to isolate and profile CTC subtypes. We thus introduced an innovative microfluidic device [5] that incorporates a hyperuniform (HU) micro-post configuration, which facilitates the isolation and potential identification of multiple CTC subtypes (see Figure 1).
The underlying hypothesis that guides this innovative approach recognizes microscale heterogeneity within the microfluidic chip. This inherent heterogeneity is thought to have the capacity to generate localized flow patterns, which lead to varying adhesive strengths — a crucial factor for the capture of CTCs at different positions within the microfluidic device. The unique design of the HU micro-post configuration seeks to enhance the precision and effectiveness of CTC isolation and offers a promising means of honing our ability to profile various CTC subtypes without the detrimental effects of traditional methods (see Figure 2).
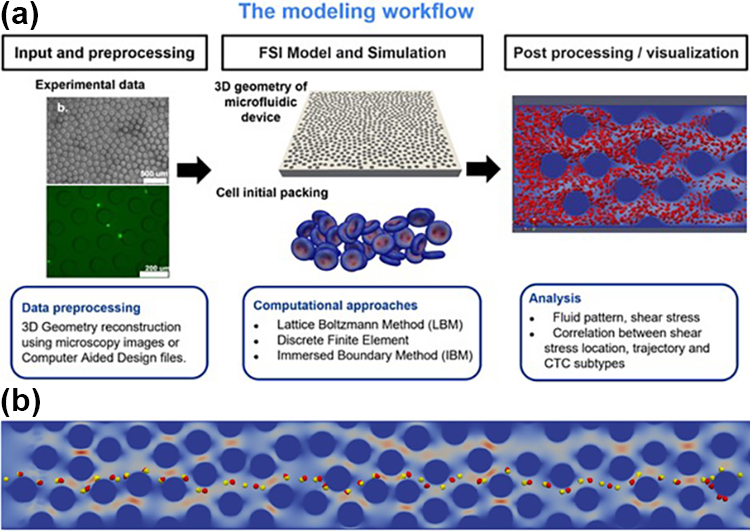
Employing a numerical approach has improved our understanding of CTC behavior and heterogeneity as we pursue our primary goal: an innovative microchip design for the label-free isolation and categorization of distinct CTC subpopulations based on their metastatic characteristics and motion dynamics within a microfluidic device. Beyond the conventional aspects of adhesive strength and physiological features—like size, elasticity, and shape—that influence CTC trajectories, our research explores the intricacies of fluid dynamics within the microfluidic device. We have crafted a cellular-scale fluid-structure interaction modeling framework that allows us to explore the complex interplay of forces and fluid dynamics within the microchip. It enables a detailed assessment of the shear stress of captured cells, thereby providing valuable insights into CTC transport and adhesion dynamics. This modeling framework progresses our comprehension of the microenvironment within the device and creates an extensive database of CTC trajectories.
Our numerical approach serves dual purposes: (i) heightening our capability to refine label-free techniques for CTC isolation and (ii) providing a foundation for the training of our artificial intelligence (AI) model. The generated database of CTC trajectories is a valuable resource for AI-driven analysis and interpretation. It may contribute to the development of a robust and intelligent platform for the noninvasive retrieval and classification of CTCs from blood samples, which could transform procedures for cancer diagnosis and treatment. The combination of innovative microchip design and sophisticated numerical modeling lays the groundwork for further advancements in our understanding of complex CTC dynamics in a microfluidic context.

Yifan Wang delivered a minisymposium presentation on this research at the 10th International Congress on Industrial and Applied Mathematics (ICIAM 2023), which took place in Tokyo, Japan, last year. He received funding to attend ICIAM 2023 through a SIAM Travel Award that was supported by U.S. National Science Foundation grant DMS-2233032. To learn more about SIAM Travel Awards and submit an application, visit the online page.
References
[1] Almendro, V., Marusyk, A., & Polyak, K. (2013). Cellular heterogeneity and molecular evolution in cancer. Annu. Rev. Pathol., 8, 277-302.
[2] Berlanda, S.F., Breitfeld, M., Dietsche, C.L., & Dittrich, P.S. (2021). Recent advances in microfluidic technology for bioanalysis and diagnostics. Anal. Chem., 93(1), 311-331.
[3] Campbell, P.J., Yachida, S., Mudie, L.J., Stephens, P.J., Pleasance, E.D., Stebbings, L.A., … Futreal, P.A. (2010). The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature, 467(7319), 1109-1113.
[4] Chinen, L.T.D. (Ed.) (2021). Circulating tumor cells: Brief overview of methods for detection. In Atlas of liquid biopsy: Circulating tumor cells and other rare cells in cancer patients’ blood (pp. 1-8). Cham, Switzerland: Springer Nature.
[5] Ding, Z., Zheng, Y., Xu, Y., Jiao, Y., & Li, W. (2018). Hyperuniform flow fields resulting from hyperuniform configurations of circular disks. Phys. Rev. E, 98(6), 063101.
[6] Dong, Z., Ahrens, C.C., Yu, D., Ding, Z., Lim, H., & Li, W. (2017). Cell isolation and recovery using hollow glass microspheres coated with nanolayered films for applications in resource-limited settings. ACS Appl. Mater. Interfaces, 9(18), 15265-15273.
[7] Navin, N., Kendall, J., Troge, J., Andrews, P., Rodgers, L., McIndoo, J., … Wigler, M. (2011). Tumour evolution inferred by single-cell sequencing. Nature, 472(7341), 90-94.
[8] Peng, J., Zhao, Q., Zheng, W., Li, W., Li, P., Zhu, L., … Yang, Y. (2017). Peptide-functionalized nanomaterials for the efficient isolation of HER2-positive circulating tumor cells. ACS Appl. Mater. Interfaces, 9(22), 18423-18428.
[9] Wen, C.-Y., Wu, L.-L., Zhang, Z.-L., Liu, Y.-L., Wei, S.-Z., Hu, J., … Pang, D.-W. (2013). Quick-response magnetic nanospheres for rapid, efficient capture and sensitive detection of circulating tumor cells. ACS Nano, 8(1), 941-949.
[10] Xu, H., Aguilar, Z.P., Yang, L., Kuang, M., Duan, H., Xiong, Y., … Wang, A. (2011). Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials, 32(36), 9758-9765.
About the Authors
Yifan Wang
Assistant professor, Texas Tech University
Yifan Wang is an assistant professor in the Department of Mathematics and Statistics at Texas Tech University. His research uses mathematical modeling and machine learning to study fluid-structure interactions in biomedical systems.

Wei Li
Associate Professor, Texas Tech University
Wei Li is an associate professor in the Department of Chemical Engineering at Texas Tech University. His lab combines cutting-edge microfluidics, soft materials, and high throughput nano-assembly techniques to develop novel functional polymer surfaces and devices for biomedical and energy applications.

Stay Up-to-Date with Email Alerts
Sign up for our monthly newsletter and emails about other topics of your choosing.



