On the Air
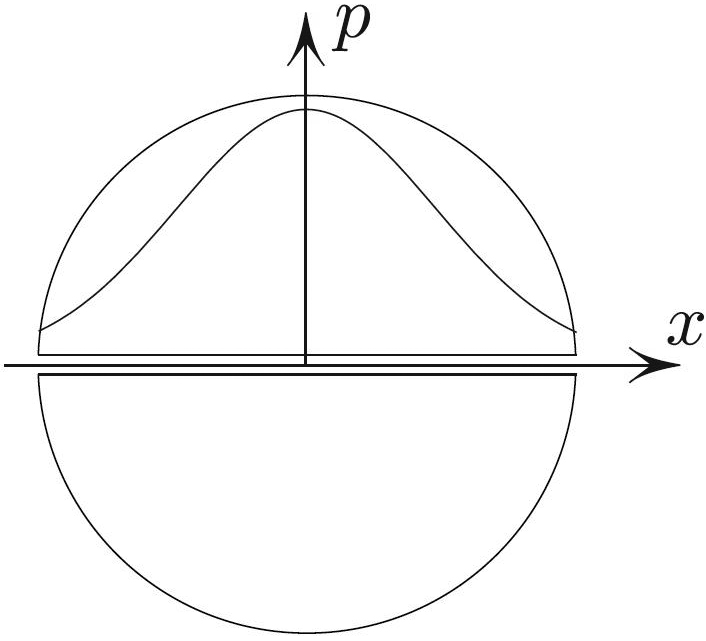
Atmospheric air pressure decreases roughly exponentially with height (with some assumptions that are neither fully realistic nor preposterous). But how would air pressure change with depth in a shaft that is drilled through the center of the Earth?1 For simplicity, let us assume the law of ideal gas for air and take the air temperature as constant. Later I will show that the pressure turns out to obey a normal distribution (see Figure 1):
\[p(x)= p(0) e^{-ax ^2 },\tag1\]
where \(x\) is the distance to the Earth’s center and
\[a= cg/2R.\]
Here, \(R\approx 6.4\cdot 10^6 \ \textrm{m}\) is the Earth’s radius, \(g\approx 10 \ \textrm{m/sec} ^2\), and \(c= \rho /p\) is the coefficient of proportionality between the density and pressure at a fixed temperature. Taking this to be room temperature, \(c\approx 10 ^{-5} \ \textrm{sec} ^2 /\textrm{m} ^2\). Substituting the expression for \(a\) into \((1)\) relates \(p(0) =p_\textrm{center}\) to \(p(R)=p_\textrm{atm}\):
\[p_\textrm{center}=p_\textrm{atm}e^{aR^2 }= p_\textrm{atm}e^{cg/2R}.\]
Substituting the values of the constants yields
\[p_\textrm{center}> p_\textrm{atm} 10^{1,000}.\tag2\]
The super-astronomical estimate is laughably large; in truth, \(p_\textrm{center}< 10 ^7 p_\textrm{atm}\). Generously speaking, my estimate \((2)\) is off by 993 orders of magnitude!
What is the source of this preposterous answer? The big mistake was to assume the ideal gas law; this law breaks down once the density reaches that of liquid gas — which then becomes almost incompressible. The air behaves like ideal gas until it doesn’t.
Gravity Is Greater Underground
Isaac Newton knew that the gravity inside a homogenous solid ball decreases linearly with depth until it reaches zero at the center. On our planet, however, the gravitational acceleration is highest not at the surface but rather at some depth — roughly halfway to the center. This is so because density is not constant and instead increases towards the center. By descending a mile underground, we gain a little bit of weight. This is especially clear in an extreme case where the entire mass of the planet is concentrated in a small concentric ball; the gravity would then be highest at the surface of the ball, which is under the planet’s surface. A related exercise for calculus students is to find the density distribution that yields constant \(g\) inside the ball.
Derivation of \((1)\)
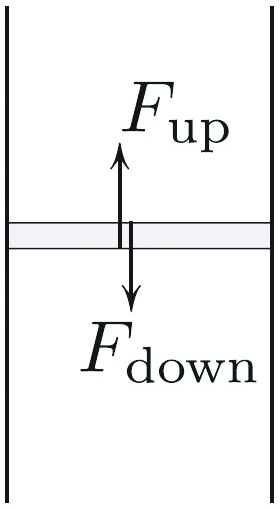
For a strip of air (see Figure 2) in the shaft to remain in equilibrium, the weight must be balanced by the excess of pressure on the bottom over that on the top:
\[F_\textrm{up}-F_\textrm{down} = \textrm{weight}.\]
Dividing both sides by the thickness \(h\) and the area of the air column’s horizontal cross-section yields
\[-p'(x) = \rho (x) g(x).\tag3\]
Here, \(g(x)\) is the gravitational acceleration in the shaft at distance \(x\) to the Earth’s center. Let us treat the Earth as a homogeneous ball; the gravity then varies linearly with \(x\), from zero to \(g\) at the surface where \(x=R\approx 6.4\cdot 10^6 \ \textrm{m}\):
\[g(x) = \frac{g}{R} x.\tag4\]
Now the density of an ideal gas at a constant temperature2 is in direct proportion to the pressure: \(\rho = c p\). Substituting this and \((4)\) into \((3)\) gives
\[p' = - 2ax\, p, \tag5\]
where \(a = cg/2R\). Separating the variables and integrating yields the normal distribution \((1)\).
A Paradox
Rising now above the ground, let me “prove” that the air pressure decreases with height — but not to zero! With the same assumptions as before and with gravity \(g(x) = \textrm{const.}/x^2\) above ground (where \(x\) is still the distance to the Earth’s center), the counterpart of \((5)\) is
\[p' = - bp/x ^2,\]
where \(b>0\) is a constant. The general solution of this equation is \(p=k e^{b/x}\) with \(k>0\). So \(p \rightarrow k>0\) as \(x \rightarrow \infty\), ostensibly proving that the air pressure in outer space is positive. Of course, what this argument really proves is that the assumptions are wrong.
1 Casting aside the impossibility of such a project.
2 If we can drill to the center of the Earth, maintaining constant temperature in the shaft will be easy by comparison.
The figures in this article were provided by the author.
About the Author
Mark Levi
Professor, Pennsylvania State University
Mark Levi (levi@math.psu.edu) is a professor of mathematics at the Pennsylvania State University.
Stay Up-to-Date with Email Alerts
Sign up for our monthly newsletter and emails about other topics of your choosing.



