Mathematics for Medicine: Changing Patients’ Lives with Systems Modeling
The full emergence of the SARS-CoV-2 pandemic in early 2020 presented a medical and scientific challenge that spanned disciplines across academia and industry. In response to this challenge, we—a team of applied mathematicians in the Quantitative Systems Pharmacology (QSP) group at Pfizer—worked alongside colleagues in other domains to understand, model, and react to the pandemic.
Pfizer’s QSP group uses mathematics (typically ordinary differential equations) to mechanistically describe the dynamics of disease and treatment. We have supported the development of novel programs for many different conditions, including type 2 diabetes, obesity, nonalcoholic steatohepatitis, cancer, and inflammatory diseases. Because medicines typically take years to advance from concept to approval, we usually generate models alongside a drug development program in a learn and confirm paradigm and utilize emerging data to continuously improve them. For SARS-CoV-2, however, it was immediately clear that historic treatment development cycles and typical modeling approaches would not be helpful. To put it simply, we—like so many others in numerous fields—had to move quickly.
As the SARS-CoV-2 pandemic gained traction, we realized that our skillset could potentially accelerate the development of effective treatments for patients. In April 2020, we thus began to build a QSP model of COVID-19 that captured both viral dynamics and immune response to infection. QSP models that support drug discovery and development follow one of two general approaches: a fit-for-purpose model or a platform model. The fit-for-purpose model focuses on a particular question, target, or mechanism but might not have much additional applicability, while the platform model is scoped to capture all pertinent aspects of a disease. In the context of COVID-19, we had to pick an approach before any information about treatment efficacy was available. As such, we wanted to be ready to address a wide range of possible model-related questions when they arose and hence decided to include viral dynamics, cellular damage, and immune response in our model.
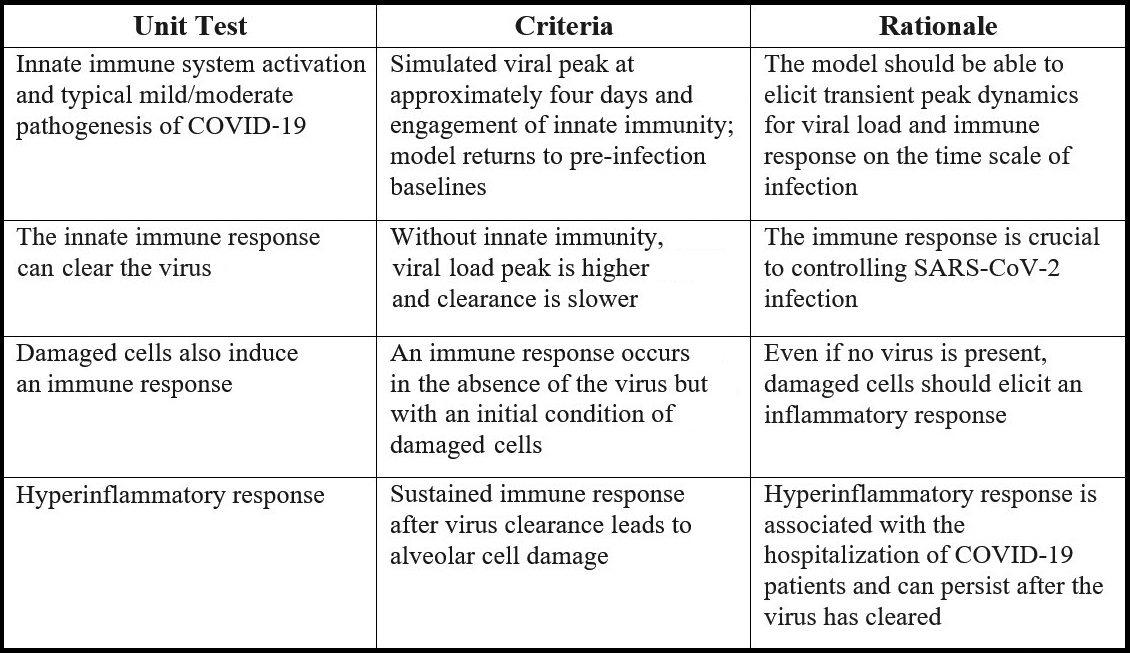
We designed the model’s desired behavior in accordance with our early understanding of viral dynamics and severe reactions to SARS-CoV-2 infection. One method of model development requires assembling relevant data and using optimization techniques to match that data, thereby ensuring that the model captures the necessary dynamics. However, such data was not accessible at the onset of the pandemic. To quickly develop the model, we defined key properties called unit tests in place of a full dataset (see Figure 1). If we could not find reasonable parameters that passed these tests, we would know that our model could not capture the observed biology and was structurally incorrect.
Our team utilized components of prior models to quickly assemble a version 0.1 of our model [2, 6, 9]. Once this model satisfied our unit tests, we established some early insights about the interplay between the strength and sensitivity of immune response versus the biomarkers of cell damage. We published our complete prototype (including all code) to bolster other comparable modeling or drug discovery/development efforts in academia or industry [4].
In theory, this model would support drug development programs that target any aspect of COVID-19. By the time our prototype version was published in full [4], a key Pfizer program was creating an antiviral therapeutic called nirmatrelvir/ritonavir that targets Mpro: a required protease in the SARS-CoV-2 replication process [7]. Once our model was calibrated to a wider array of data, it could make several key predictions with a clear impact on the possible pursuit of this investigational therapeutic and the optimization of clinical trial design [8]. Figure 2 summarizes the early questions that influenced our model and its ultimate outcomes.
![<strong>Figure 2.</strong> Key predictions of the COVID-19 model and comparison to outcomes in the EPIC-HR clinical trial [5]. Figure courtesy of the authors.](/media/oiakyzxj/figure2.jpg)
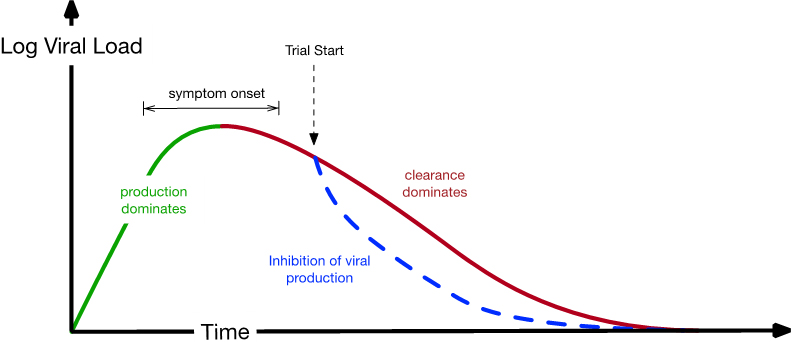
One key question is as follows: At what point after initial infection (in clinical trials and real life) should patients start taking medication? Based on other clinical trial data and the incubation period of SARS-CoV-2, we were confident that this point would usually occur post-peak viral load (see Figure 3). The first query in Figure 2 may appear straightforward, but some situations (and models) exhibit target-cell limited dynamics where viral load peaks when virion production ceases due to the infection of all potential target cells. In this scenario, a medication that inhibits viral production cannot be efficacious because there is no viral production; in Figure 3, a lack of production would occur at the “clearance dominates” portion of the curve. Our unit test guaranteed that this circumstance was not necessarily true for our model. Once it was calibrated with data, the model clearly predicted that COVID-19’s peak was driven by a balance of production and clearance of the virus, with immune response driving the latter. Our model also predicted that five days of treatment (and the inhibition of viral production) sufficiently allows the immune system to clear the remainder of the virus in nearly all patients.
Of course, this is not to say that we got everything right. We slightly overpredicted the amount by which the viral load would decrease (see Figure 2), as translating in vitro drug potency to clinical potency is always challenging. In this case, the changing nature of the COVID-19 pandemic (e.g., the emergence of new variants) and an increased prevalence of seropositivity meant that our clinical trial participants had lower baseline viral loads when they entered the trial than we expected (based on older trials). In both our model and real life, these factors are correlated with reduced viral load, which lowers treatment efficacy.
We were least confident in our prediction of the relative risk reduction of hospitalization or death for treated patients versus the placebo group. This uncertainty stemmed partly from the fact that the methodology was based on observational data of interleukin-6 levels in patients; we did not know whether this relationship would hold in treated clinical trial participants and/or cohorts with large variability in response to infection. But excitingly, our model predicted that the medication would be highly efficacious in preventing hospitalization — a result that only increased our anticipation of the clinical trial’s outcome.
Learning that the investigational therapeutic was efficacious [5] was certainly a career highlight for everyone involved. It was incredibly gratifying to know that our model predictions, to a very large degree, were borne out in the clinical trial. However, our story does not end there. With this pivotal data in hand, we updated the model to precisely match the clinical trial and used it to inform discussions with regulatory agencies about this promising investigational therapeutic. For example, we shared our model with the U.S. Food and Drug Administration to support their review [3] and subsequent approval of nirmatrelvir/ritonavir tablets (commercially known as PAXLOVID™).
To enable robust predictions, we developed representative virtual populations [1, 8] whose viral dynamics and risk of hospitalization or death (predicted based on immune response) matched available data. While constructing these virtual populations, we realized that some virtual patients in both untreated and treated groups would often experience a second viral load peak after a certain time period. We could not identify a scientific reason for excluding these patients from our virtual populations and thus retained them, even though we were unsure of the likelihood of observation. As more data emerged, we began to comprehend that this phenomenon—now known as viral load rebound—is very real. Moreover, we could use the model to understand its relative occurrence in different populations (e.g., immunocompromised patients) and the impact of longer dosing.
Accelerating the development of novel medications while simultaneously generating appropriate safety and efficacy data and maintaining regulatory compliance is undoubtedly difficult. Building a QSP model in the midst of a global pandemic was sometimes stressful, sometimes frustrating, and sometimes of uncertain value. We therefore greatly appreciate the support and collaboration of our many colleagues who worked tirelessly and innovatively to mitigate and ultimately remove all obstacles and make nirmatrelvir/ritonavir (commercially knowns as PAXLOVID™) available to eligible patients. Our positive outcome serves as a broad reminder to trust your models and listen to the mathematics.
PAXLOVID has been approved for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. PAXLOVID has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in pediatric patients (12 years of age and older weighing at least 40 kg) who are at high risk for progression to severe COVID-19, including hospitalization or death; and the emergency use of PAXLOVID is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner. For additional information, please refer to the product’s U.S. Prescribing Information (including BOXED WARNING).
References
[1] Allen, R.J., Rieger, T.R., & Musante, C.J. (2016). Efficient generation and selection of virtual populations in quantitative systems pharmacology models. CPT Pharmacometrics Syst. Pharmacol., 5(3), 140-146.
[2] Baccam, P., Beauchemin, C., Macken, C.A., Hayden, F.G., & Perelson, A.S. (2006). Kinetics of influenza A virus infection in humans. J. Virol., 80(15), 7590-7599.
[3] Center for Drug Evaluation and Research. (2023). Application number: 21788Orig1s000 integrated review. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217188Orig1s000IntegratedR.pdf.
[4] Dai, W., Rao, R., Sher, A., Tania, N., Musante, C.J., & Allen, R. (2021). A prototype QSP model of the immune response to SARS-CoV-2 for community development. CPT Pharmacometrics Syst. Pharmacol., 10(1), 18-29.
[5] Hammond, J., Leister-Tebbe, H., Gardner, A., Abreu, P., Bao, W., Wisemandle, W., … Rusnak, J.M. (2022). Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med., 386(15), 1397-1408.
[6] Lee, H.Y., Topham, D.J., Park, S.Y., Hollenbaugh, J., Treanor, J., Mosmann, T.R., … Wu, H. (2009). Simulation and prediction of the adaptive immune response to influenza A virus infection. J. Virol., 83(14), 7151-7165.
[7] Owen, D.R., Allerton, C.M.N., Anderson, A.S., Aschenbrenner, L., Avery, M., Berritt, S., … Zhu, Y. (2021). An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science, 374(6575), 1586-1593.
[8] Rao, R., Musante, C.J., & Allen, R. (2023). A quantitative systems pharmacology model of the pathophysiology and treatment of COVID-19 predicts optimal timing of pharmacological interventions. NPJ Syst. Biol. Appl., 9(1), 13.
[9] Rogers, K.V., Martin, S.W., Bhattacharya, I., Singh, R.S.P., & Nayak, S. (2021). A dynamic quantitative systems pharmacology model of inflammatory bowel disease: Part 1 – model framework. Clin. Transl. Sci., 14(1), 239-248.
About the Authors
Richard Allen
Pfizer
Richard Allen has been supporting drug discovery and development with mechanistic modeling at Pfizer for the past 12 years. He holds a Ph.D. in applied mathematics from University College London and was a postdoctoral fellow at the University of North Carolina at Chapel Hill.
Rohit Rao
Pfizer
Rohit Rao has been supporting mechanistic modeling efforts at Pfizer for the past five years. He holds a Ph.D. in chemical and biochemical engineering from Rutgers University and completed a postdoctoral fellowship at Harvard University.
C.J. Musante
Pfizer
C.J. Musante has been supporting drug discovery and development with mechanistic modeling at Pfizer for the past 15 years. She holds a Ph.D. in applied mathematics from North Carolina State University and was a postdoctoral fellow at the University of North Carolina at Chapel Hill.
Related Reading
Stay Up-to-Date with Email Alerts
Sign up for our monthly newsletter and emails about other topics of your choosing.